Abstract
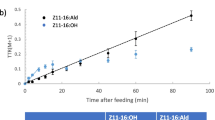
4,8-Dimethyldecanal (4,8-DMD) is the aggregation pheromone produced by male red flour beetles (RFB), Tribolium castaneum. To elucidate the biosynthetic origin of 4,8-DMD, the following studies were performed: (1)effects of juvenile hormone (JH) III, and pathway inhibitors mevastatin, an inhibitor of the mevalonate pathway, and 2-octynoic acid, an inhibitor of the fatty acid pathway, were tested to determine whether 4,8-DMD is derived from the fatty acid pathway or the mevalonate pathway; (2) incorporation of 13C-labeled acetate, propionate, and mevalonolactone into 4,8-DMD was measured to directly determine the biosynthetic origin of 4,8-DMD; and (3) incorporation of deuterium-labeled precursors, including 2-methylbutanoate (C5D), 4-methylhexanoate (C7D), 2,6-dimethyloctanoate (C10D), and 4,8-dimethyldecanoate (C12D) was tested to determine whether 4,8-DMD is biosynthesized in the sequence AcPrAcPrAc (Ac; acetate, Pr; propionate). JH III was topically applied to males at various doses. Inhibitors and isotopically labeled substrates were administered orally by feeding the beetles flour treated with the substrates of interest, after which volatiles were collected from both sexes of RFBs. The amount of 4,8-DMD produced was significantly reduced with increasing doses of JH III. Also, 2-octynoic acid inhibited the production of 4,8-DMD, but mevastatin did not. Exposure of RFBs to [1-13C]acetate and [1-13C]propionate, but not [2-13C]mevalonolactone, resulted in incorporation of the labeled compounds into 4,8-DMD. RFBs fed flour treated with deuterium-labeled C5D, C10D, and C12D, but not C7D, incorporated these compounds into 4,8-DMD. The findings that the production of 4,8-DMD was inhibited by 2-octynoic acid but unaffected by mevastatin, combined with the high incorporation of [1-13C]acetate and [1-13C]propionate into 4,8-DMD and the incorporation of deuterated precursors, unambiguously demonstrated that 4,8-DMD is of fatty acid rather than terpene biosynthetic origin, and that the biosynthesis of 4,8-DMD proceeds in the sequence Ac-Pr-Ac-Pr-Ac.








Similar content being viewed by others
References
L. S. Barkawi W. Francke G. J. Blomquist S. J. Seybold (2003) ArticleTitleFrontalin: De novo biosynthesis of an aggregation pheromone component by Dendroctonus spp. bark beetles (Coleoptera: Scolytidae) Insect Biochem. Mol. Biol. 33 773–788 Occurrence Handle10.1016/S0965-1748(03)00069-9 Occurrence Handle1:CAS:528:DC%2BD3sXlsVSntbY%3D Occurrence Handle12878224
R. J. Bartelt D. Weisleder (1996) ArticleTitlePolyketide origin of pheromones of Carpophilus davidsoni and C. mutilatus (Coleoptera: Nitidulidae) Bioorg. Med. Chem. 4 429–438 Occurrence Handle10.1016/0968-0896(96)00022-3 Occurrence Handle1:CAS:528:DyaK28Xisl2mu7w%3D Occurrence Handle8733623
R. Beier B. P. Mundy (1979) ArticleTitleA facile removal of the tetrahydropyranyl protecting group from alcohol derivatives Synth. Commun. 9 271–273 Occurrence Handle1:CAS:528:DyaE1MXktVGjtLY%3D
I. M. Campbell (1974) ArticleTitleIncorporation and dilution values—their calculation in mass spectrally assayed stable isotope labeling experiments Bioorg. Chem. 3 386–387 Occurrence Handle10.1016/0045-2068(74)90010-8 Occurrence Handle1:CAS:528:DyaE2MXot1CjtA%3D%3D
J. Chase K. Touhara G. D. Prestwich C. Schal G. J. Blomquist (1992) ArticleTitleBiosynthesis and endocrine control of the production of the German cockroach sex pheromone 3,11-dimethylnonacosan-2-one Proc. Natl. Acad. Sci. U.S.A. 89 6050–6054 Occurrence Handle1:CAS:528:DyaK38Xlt1ynsLk%3D Occurrence Handle1631090
N. M. Chen J. H. Borden H. D. Pierce SuffixJr (1988) ArticleTitleEffect of juvenile hormone analog, fenoxycarb, on pheromone production by Ips paraconfusus (Coleoptera: Scolytidae) J. Chem. Ecol. 14 1087–1098 Occurrence Handle10.1007/BF01019337 Occurrence Handle1:CAS:528:DyaL1cXktFyrsL4%3D
J. W. Dillwith J. H. Nelson J. G. Pomonis D. R. Nelson G. J. Blomquist (1982) ArticleTitleA 13C NMR study of methyl-branched hydrocarbon biosynthesis in the housefly J. Biol. Chem. 257 11305–11314 Occurrence Handle1:CAS:528:DyaL38Xlt1CntrY%3D Occurrence Handle7118886
A. Endo M. Kuroda K. Tanzawa (1976) ArticleTitleCompetitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-36A and ML-236B fungal metabolites, having hypocholesterolemic activity FEBS Lett. 72 323–326 Occurrence Handle10.1016/0014-5793(76)80996-9 Occurrence Handle1:CAS:528:DyaE2sXosFartw%3D%3D
D. L. Faustini W. E. Burkholder R. J. Laub (1981) ArticleTitleSexually dimorphic setiferous sex patch in the male red flour beetles Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae): site of aggregation pheromone production J. Chem. Ecol. 7 465–480 Occurrence Handle10.1007/BF00995769 Occurrence Handle1:CAS:528:DyaL3MXksFynurs%3D
K. Freund J. Mizzer W. Dick C. Thorpe (1985) ArticleTitleInactivation of general acyl-CoA dehydrogenase from pig kidney by 2-alkynoyl coenzyme A derivatives: Initial aspects Biochemistry 24 5996–6002 Occurrence Handle10.1021/bi00342a046 Occurrence Handle1:CAS:528:DyaL2MXlsFKhurk%3D Occurrence Handle4084503
R. P. Hanzlik (1988) Selective epoxidation of terminal double bonds: 10,11-Epoxyfarnesyl acetate W. E. Noland (Eds) Organic Syntheses Coll. Vol. 6 Wiley New York 560–564
P. E. Howse O. T. Jones I. D. R. Stevens (1998) Insect Pheromones and Their Use in Pest Management Chapman & Hall London, UK
Hussain, A. 1993. Chemical ecology of Tribolium castaneum Herbst (Coleoptera: Tenebrionidae): Factors affecting biology and applications of pheromone. Ph.D. dissertation. Oregon State University.
N. Islam R. Bacala A. Moore D. Vanderwel (1999) ArticleTitleBiosynthesis of 4-methyl-1-nonanol: Female-produced sex pheromone of the yellow mealworm beetle, Tenebrio molitor (Coleoptera: Tenebrionidae) Insect Biochem. Mol. Biol. 29 201–208 Occurrence Handle10.1016/S0965-1748(98)00126-X Occurrence Handle1:CAS:528:DyaK1MXjtVaru7o%3D
P. Ivarsson G. Birgersson (1995) ArticleTitleRegulation and biosynthesis of pheromone components in the double spined bark beetle Ips duplicatus (Coleoptera: Scolytidae) J. Insect Physiol. 41 840–843 Occurrence Handle10.1016/0022-1910(95)00052-V
P. Ivarsson F. Schlyter G. Birgersson (1993) ArticleTitleDemonstration of de novo pheromone biosynthesis in Ips duplicatus (Coleoptera: Scolytidae): Inhibition of ipsdienol and E-myrcenol production by compactin Insect Biochem. Mol. Biol. 23 655–662 Occurrence Handle10.1016/0965-1748(93)90039-U Occurrence Handle1:CAS:528:DyaK3sXlslOrt78%3D
R. A. Jurenka (2003) Biochemistry of female moth sex pheromones G. J. Blomquisst R. G. Vogt (Eds) Insect Pheromone Biochemistry and Molecular Biology Elsevier London, UK 53–80
R. Jurenka (2004) Insect pheromone biosynthesis S. Schulz (Eds) The Chemistry of Pheromones and Other Semiochemicals. I. Topics in Current Chemistry, Vol. 239 Springer-Verlag Heidelberg, Germany 97–132
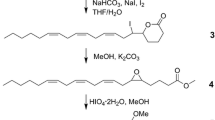
J. Kim S. Matsuyama T. Suzuki (2004) ArticleTitleDeuterated analogues of 4,8-dimethyldecanal, the aggregation pheromone of Tribolium castaneum: synthesis and pheromonal activity J. Label. Compd. Radiopharm. 47 921–934 Occurrence Handle10.1002/jlcr.881 Occurrence Handle1:CAS:528:DC%2BD2cXhtFajtbjN
Lawson, J. A., Cowell, W. T., DeGraw, J. I., Peters, R. H., Dehn, R. L., and Tanabe, M. 1975. The synthesis of singly and double 13C-labeled mevalonolactone. Synthesis 729–730.
A. L. Lehninger D. L. Nelson M. M. Cox (1993) Principles of Biochemistry EditionNumber2 Worth Publishers New York, NY
D. Monger W. A. Lim F. J. Kézdy J. H. Law (1982) ArticleTitleCompactin inhibits insect HMG-CoA reductase and juvenile hormone biosynthesis Biochem. Biophys. Res. Commun. 105 1374–1380 Occurrence Handle10.1016/0006-291X(82)90939-1 Occurrence Handle1:CAS:528:DyaL38XitFams74%3D Occurrence Handle7103961
H. Nagano T. Nagasawa M. Sakuma (1997) ArticleTitleSynthesis of 2-polyprenyl-substituted polyprenols and their conversion into phosphates Bull. Chem. Soc. Jpn. 70 1969–1976 Occurrence Handle1:CAS:528:DyaK2sXlsVCnsro%3D
H. Nagano E. Nakanishi S. Takajo M. Sakuma K. Kudo (1999) ArticleTitleSynthesis of 6-(poly)prenyl-substituted polyprenols and their phosphates Tetrahedron 55 2591–2608 Occurrence Handle10.1016/S0040-4020(99)00057-5 Occurrence Handle1:CAS:528:DyaK1MXhs1WqsLo%3D
D. R. Nelson (1993) Methyl-branched lipids in insects D. W. Stanley-Samuelson D. R. Nelson (Eds) Insect Lipids: Chemistry, Biochemistry, and Biology University of Nebraska Press Lincoln, NE 271–315
A. M. Pierce H. D. Pierce SuffixJr J. H. Borden A. C. Oehlschlager (1986) ArticleTitleEnhanced production of aggregation pheromones in four stored-product coleopterans feeding on methoprene-treated oats Experientia 42 164–165 Occurrence Handle1:CAS:528:DyaL28XhvVWks70%3D
M. C. B. Qazi C. R. B. Boake S. M. Lewis (1998) ArticleTitleThe femoral setiferous glands of Tribolium castaneum males and production of the pheromone 4,8-dimethyldecanal Entomol. Exp. Appl. 89 313–317 Occurrence Handle10.1046/j.1570-7458.1998.00414.x Occurrence Handle1:CAS:528:DyaK1MXivFSrurY%3D
G. A. Ropp (1950) ArticleTitlePreparation of ethyl acetate-2-14C and n-butyl acetate-2-14C using alkyl phosphates J. Am. Chem. Soc. 72 2299–2317 Occurrence Handle10.1021/ja01161a526 Occurrence Handle1:CAS:528:DyaG3cXktlGltQ%3D%3D
S. J. Seybold D. Vanderwel (2003) Biosynthesis and endocrine regulation of pheromone production in the Coleoptera G. J. Blomquist R. G. Vogt (Eds) Insect Pheromone Biochemistry and Molecular Biology Elsevier London, UK 137–200
R. R. Sokal F. J. Rohlf (1995) Biometry EditionNumber3 Freeman New York, NY
T. Suzuki (1980) ArticleTitle4,8-Dimethyldecanal: The aggregation pheromone of the flour beetles, Tribolium castaneum and T. confusum (Coleoptera: Tenebrionidae) Agric. Biol. Chem. 44 2519–2520 Occurrence Handle1:CAS:528:DyaL3MXhtlSgtQ%3D%3D
T. Suzuki K. Mori (1983) ArticleTitle(4R,8R)-()-4,8-Dimethyldecanal: the natural aggregation pheromone of the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae) Appl. Entomol. Zool. 18 134–136 Occurrence Handle1:CAS:528:DyaL3sXitFeisLk%3D
T. Suzuki H. Nakakita Y. Kuwahara (1987) ArticleTitleAggregation pheromone of Tribolium freemani Hinton (Coleoptera, Tenebrionidae): I. Identification of the aggregation pheromone Appl. Entomol. Zool. 22 340–347 Occurrence Handle1:CAS:528:DyaL2sXmtFKns7c%3D
Y. Tanaka H. Honda K. Ohsawa I. Yamamoto (1986) ArticleTitleA sex attractant of the yellow mealworm, Tenebrio molitor L., and its role in mating behaviour J. Pestic. Sci. 11 49–55 Occurrence Handle1:CAS:528:DyaL28XksFyitb8%3D
Y. Tanaka H. Honda K. Ohsawa I. Yamamoto (1989) ArticleTitleAbsolute configuration of 4-methyl-1-nonanol, a sex attractant of the yellow mealworm, Tenebrio molitor L J. Pestic. Sci. 14 197–202 Occurrence Handle1:CAS:528:DyaL1MXlvV2msLo%3D
T. Tashiro S. Kurosawa K. Mori (2004) ArticleTitleRevision of the structure of the major aggregation pheromone of the broad-horned flour beetle (Gnatocerus cornutus) to (1S,4R,5R)-alpha-acoradiene by its synthesis Biosci. Biotechnol. Biochem. 68 663–670 Occurrence Handle10.1271/bbb.68.663 Occurrence Handle1:CAS:528:DC%2BD2cXjt1OktLc%3D Occurrence Handle15056901
S. Tebayashi N. Hirai T. Suzuki S. Matsuyama H. Nakakita T. Nemoto H. Nakanishi (1998a) ArticleTitleIdentification of (+)-acoradiene as an aggregation pheromone for Gnatocerus cornutus (F.) (Coleoptera: Tenebrionidae) J. Stored Prod. Res. 34 99–106 Occurrence Handle10.1016/S0022-474X(98)00004-6 Occurrence Handle1:CAS:528:DyaK1cXks1KhsL4%3D
S. Tebayashi N. Hirai T. Suzuki S. Matsuyama H. Nakakita T. Nemoto H. Nakanishi (1998b) ArticleTitleα-Cedren-14-al: minor aggregation pheromone component of Gnatocerus cornutus (F.) (Coleoptera: Tenebrionidae) J. Pestic. Sci. 23 402–406 Occurrence Handle1:CAS:528:DyaK1cXnvFSqurw%3D
J. A. Tillman S. J. Seybold R. A. Jurenka G. J. Blomquist (1999) ArticleTitleInsect pheromonesan overview of biosynthesis and endocrine regulation Insect Biochem. Mol. Biol. 29 481–514 Occurrence Handle10.1016/S0965-1748(99)00016-8 Occurrence Handle1:CAS:528:DyaK1MXksVehu7k%3D Occurrence Handle10406089
C. Tittiger (2003) Molecular biology of bark beetle pheromone production and endocrine regulation G. J. Blomquist R. G. Vogt (Eds) Insect Pheromone Biochemistry and Molecular Biology Elsevier London, UK 201–230
D. Vanderwel A. C. Oehlschlager (1987) Biosynthesis of pheromones and endocrine regulation of pheromone production in Coleoptera G. D. Prestwich G. J. Blomquist (Eds) Pheromone Biochemistry Academic Press Orlando, FL 175–215
Vanderwel, D., Pierce, H. D. Jr., Oehlschlager, A. C., Borden, J. H., Pierce, A. M. 1990. Macrolide (cucujolide) biosynthesis in the rusty grain beetle, Cryptolestes ferrugineus I. Insect Biochem. 20:567–572.
D. Vanderwel B. Johnson A. C. Oehlschlager (1992) ArticleTitleCucujolide biosynthesis in the merchant and rusty grain beetles Insect Biochem. Mol. Biol. 22 875–883 Occurrence Handle10.1016/0965-1748(92)90114-T Occurrence Handle1:CAS:528:DyaK3sXisFWltLg%3D
C.-H. Zhao R. O. Adlof C. Löfstedt (2004) ArticleTitleSex pheromone biosynthesis in the pine caterpillar moth, Dendrolimus punctatus (Lepidoptera: Lasiocampidae): pathway leading to Z5-monoene and 5,7-conjugated diene components Insect Biochem. Mol. Biol. 34 261–271 Occurrence Handle10.1016/j.ibmb.2003.10.005 Occurrence Handle1:CAS:528:DC%2BD2cXhtFWis7c%3D Occurrence Handle14871622
Acknowledgment
This work was partially supported by the Iijima Memorial Foundation for the Promotion of Food Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Matsuyama, S. & Suzuki, T. 4,8-Dimethyldecanal, the Aggregation Pheromone of Tribolium castaneum, is Biosynthesized Through the Fatty Acid Pathway. J Chem Ecol 31, 1381–1400 (2005). https://doi.org/10.1007/s10886-005-5292-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-5292-3